
Connections between maintenance of diversity and variation in nature
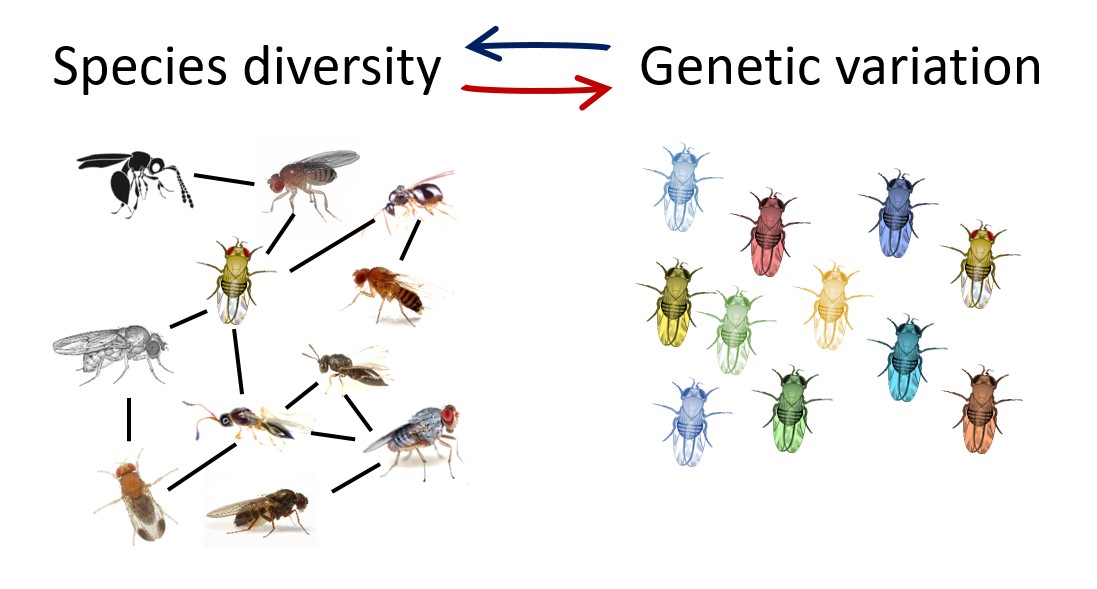 How do species coexist in diverse ecological networks despite competition, predation and parasitism? This is the most enigmatic question in community ecology, with an analogy at the population level: How is genetic variation maintained within species despite selection and drift? These twin questions are typically studied separately.
How do species coexist in diverse ecological networks despite competition, predation and parasitism? This is the most enigmatic question in community ecology, with an analogy at the population level: How is genetic variation maintained within species despite selection and drift? These twin questions are typically studied separately.
But evidence for rapid evolution of traits within a few generations is accumulating, meaning that ecological and evolutionary processes are tightly interconnected in a single process, usually called eco-evolutionary dynamics.

We are interested in understanding rapid evolution in species interactions and in experimentally testing feedback loop between maintenance of diversity in communities and variation in populations. We use a nicely tractable network of tropical rainforest Drosophila and their parasitoids (6 fly and 5 wasp species), which allows multigenerational microcosm experiments. To link the findings to natural eco-evolutionary dynamics, we investigate mechanisms maintaining diversity and variation in the wild.
The project is funded by ERC Consolidator grant (2024-2028)
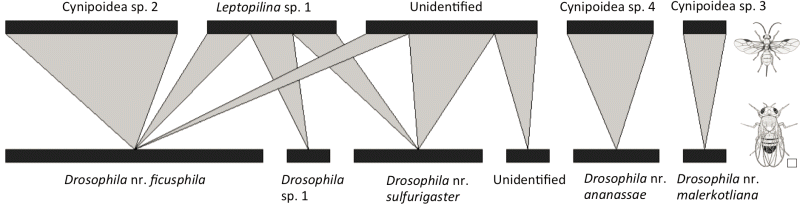 Abiotic and biotic factors structuring food webs
Abiotic and biotic factors structuring food webs
This project utilizes a novel food web model system of wild Drosophila species and their parasitoids to answer fundamental questions in community ecology. We are surveying the focal food webs along environmental gradients in tropical Australia and Papua New Guinea using modern DNA metabarcoding methods and disentangling the factors structuring the food webs using field and laboratory experiments. We especially focus on the effect of warming on communities.
Pardikes, N.A., Revilla, T., Lue, C-H., Thierry, M., Souto-Vilarós, D. & Hrcek, J. (2022) Effects of phenological mismatch under warming are modified by community context.
Global Change Biology 28, 4013-4026.
Thierry, M., Pardikes, N.A., Rosenbaum, B. Ximénez-Embún M.G. & Hrcek, J. (2022) The presence of multiple parasitoids decreases host survival under warming, but parasitoid performance also decreases. Proceedings of the Royal Society B 289, 20220121.
Jeffs, C.T., Terry, J.C.D., Higgie, M., Jandová, A., Konvicková, H., Brown, J.J., Lue, C-H., Schiffer M., O'Brien E.K., Bridle, J., Hrcek, J. & Lewis, O.T. (2021) Molecular analyses reveal consistent food web structure with elevation in rainforest Drosophila – parasitoid communities. Ecography 44, 403-413.
Thierry, M., Hrcek, J. & Lewis, O.T. (2019) Mechanisms structuring host-parasitoid networks in a global warming context: a review. Ecological Entomology, 44: 581-592.
 The role of microbial symbionts in food webs
The role of microbial symbionts in food webs
Bacterial symbionts of insects are abundant and diverse, and have numerous effects on their hosts' biology. We have recently described Drosophila microbiome structure along tropica elevational gradient. We have also shown that symbiotic bacteria protect aphids against parasitoids and pathogens under natural field conditions.
Brown, J.J., Jandová, A., Jeffs C.T., Higgie, M., Nováková, E., Lewis, O.T., Hrcek, J. (2023)
Microbiome structure of a wild Drosophila community along tropical elevational gradients and comparison to laboratory lines Applied and Environmental Microbiology, 89 (5), e00099-23.
Brown, J.J., Mihaljevic, J.R., Des Marteaux, L. & Hrcek, J. (2020) Metacommunity theory for transmission of heritable symbionts within insect communities Ecology and Evolution, 10: 1703-1721.
Hrcek, J., Parker, B.J., McLean, A.H.C., Simon, J-C., Mann, C.M. & Godfray, H.C.J. (2018) Hosts do not simply outsource pathogen
resistance to protective symbionts. Evolution, 72: 1488–1499.
Hrcek, J., McLean, A.H.C. & Godfray, H.C.J. (2016) Symbionts modify interactions between insects and natural enemies in the field. Journal of Animal Ecology, 85: 1605–1612.
McLean, A.H.C., Parker, B.J., Hrcek, J., Henry, L.M. & Godfray, H.C.J. (2016) Insect symbionts in food webs. Philosophical Transactions B, doi: 10.1098/rstb.2015.0325
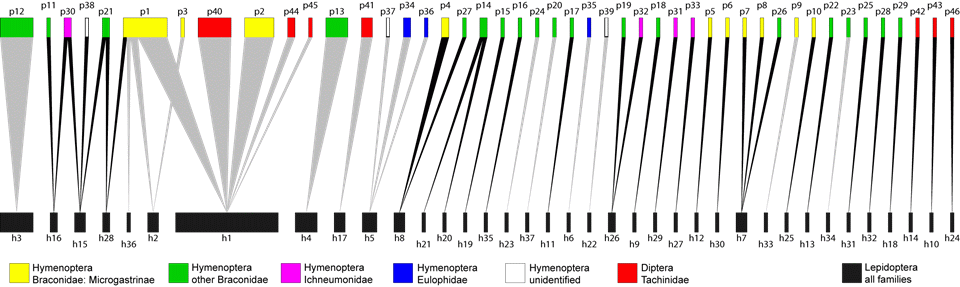 Molecular food web reconstruction
Molecular food web reconstruction
We are developing new methods for parasitoid detection in the context of species-rich food webs. We want to provides a general approach, allowing the detection and identification of parasitoids without a priori expectations on which species could be present. The parasitoid identification makes use of DNA barcoding and metabarcoding for rapid species identification, revolutionizing the way food webs are surveyed. We concentrate on methodological as well as conceptual advances.
Lue, C-H., Abram, P.K., Hrcek, J., Buffington, M.L., Staniczenko, P.P.A. (2023) Metabarcoding and applied ecology with hyper-diverse organisms: recommendations for biological control research. Molecular Ecology
Lue, C-H. et al. (2021) DROP: Molecular voucher database for identification of Drosophila parasitoids. Molecular Ecology Resources 21, 2437-2454.
Hrcek, J. & Godfray, H.C.J. (2015) What do molecular methods bring to host–parasitoid food webs? Trends in Parasitology, 31: 30-35.
Hrcek, J., Miller, S.E., Quicke, D.L.J., & Smith, M.A. (2011) Molecular detection of trophic links in a complex insect host-parasitoid food web. Molecular Ecology Resources, 11: 786-794.
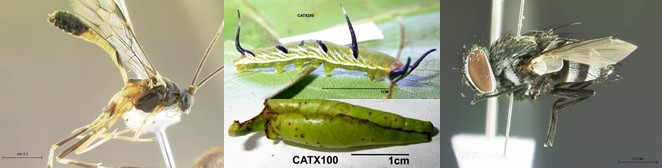
Tropical caterpillar-parasitoid food webs
We have described and analyzed a large food web of caterpillars and their parasitoids from New Guinea rainforest. The work has uncovered a species-rich community of specialized parasitoids attacking underappreciated caterpillars which hide in rolled or tied leaves. We have used a combination of traditional taxonomy, coordinating the work of multiple taxonomists, with DNA barcoding approach. The project has sprouted large number of collaborations which have built on different aspects of the data for global scale food web pattern synthesis and insight into ecological niche concept, and allowed modern parasitoid taxonomy with valuable host records.
Hrcek, J., Miller, S.E., Whitfield, J.B., Shima, H. & Novotny, V. (2013) Parasitism rate, parasitoid community composition and host specificity on exposed and semi-concealed caterpillars from a tropical rainforest. Oecologia, 173: 521–532.
Forister, M.L. et al. (2015) The global distribution of diet breadth in
insect herbivores. PNAS, 112: 442-447.
Quicke, D.L.J., Belokobylskij, S.A., Smith, M.A., Rota, J., Hrcek, J. & Butcher, B.A. (2016) A new genus of rhysipoline wasp (Hymenoptera: Braconidae) with modified wing venation from Africa and Papua New Guinea, parasitoid on Choreutidae (Lepidoptera). Annales Zoologici, 66: 173-192.